Human eyes can see all kinds of colors in the visible light band, and ears can hear sounds in the frequency range of 20-20000 Hz, but we still don’t know whether we have the sense of sensing the earth’s magnetic field, which is a complete mystery.
The public calls it "the sixth sense", but scientists don’t like this statement. In the past few decades, they have been trying to pry open the tip of the iceberg of the mystery and find a breakthrough in birds. How do they realize the induction of geomagnetic field and use it? European Robin is an excellent research object. This nocturnal migratory bird has been proved to have the ability of geomagnetic navigation before, and they can use the geomagnetic field as weak as 0.5 gauss for long-distance orientation to complete the seasonal migration year after year.

But what is the mechanism behind it? For this question, it needs a highly interdisciplinary cooperation to answer one or two. At 23: 00 on June 23rd, Beijing time, the top academic journal Nature published a heavy research titled "Magnetic sensitivity of cryptochrome 4 from a migratory songbird" on the cover form, which was jointly completed by scientists from Germany’s Oldenburg University, England’s Oxford University, China Academy of Sciences’ Hefei Institute of Material Science, Purdue University, University of Texas Southwest Medical Center and Germany’s Freiburg University. Studies have proved that cryptochrome protein (Cry4) in birds’ retina is very sensitive to magnetic field, and it may be the long-sought magnetic sensor.
There are 6 corresponding authors in this study, one of whom is Xie Can, a researcher from the High Magnetic Field Science Center of Hefei Institute of Material Science, China Academy of Sciences, and the High Magnetic Field Science Center is the common communication unit. Together with the team of the University of Oldenburg, Xie Can’s laboratory successfully prepared and obtained the Cry protein of European robin, a nocturnal migratory bird, for the first time in the laboratory, which laid the foundation for the success of the whole study.

In an interview with The Paper (www.thepaper.cn), Xie Can said that this multi-party cooperation, which started in 2016, is only the first stage of research on biomagnetic induction in various laboratories. "This article is also the first stage of our long-term cooperation." There is still a lot of work to be explored by its laboratories and partners in the future.
Xie Can graduated from the Institute of Genetics and Developmental Biology, Chinese Academy of Sciences in 2001 with a doctorate in science. In August of the same year, he went to the United States to study molecular biophysics and structural biology in Timothy Springer laboratory of Harvard Medical School. From 2009 to 2019, he was a professor at Peking University College of Life Sciences. In November 2019, he was invited to join and transferred to the High Magnetic Field Science Center of China Academy of Sciences as a researcher. In December, 2020, we jointly established the International Magnetic Biology Frontier Research Center (IMF RC).
It is worth mentioning that in 2016, Xie Can Laboratory published its original breakthrough in the field of animal magnetic induction and biological navigation in the international academic journal Nature Materials, and discovered the magnetic receptor gene for animals’ perception of magnetic field for the first time in the world. The magnetic induction protein (MagR) encoded by this gene has intrinsic magnetism and can form a complex with Cry protein, so as to recognize the external magnetic field and respond. Based on this, Xie can put forward the hypothesis of "biological compass" for animals to feel magnetism. This work was rated as "Top Ten Progress in Life Sciences in China in 2015".
Professor Peter Hore from the Laboratory of Physical and Theoretical Chemistry of the Department of Chemistry of Oxford University is also the corresponding author of this latest study. Hore is one of the main schools of biological magnetic induction hypothesis, that is, the main contributor to the chemical radical-pair model that relies on cryptochrome. In an email to The Paper (www.thepaper.cn), he wrote, "If we can prove that Cry4 is a magnetic receptor molecule, we will also prove a fundamental quantum mechanism, which enables animals to perceive extremely weak environmental stimuli, and the intensity of this stimulus is one million times weaker than previously thought".
Referring to the above hypothesis of chemical free radicals, the time should be set back to 1978. At that time, German chemist Klaus Schulten and others proposed a preliminary model to introduce quantum entanglement into this research field, arguing that the magnetic induction mechanism of birds involves the entangled state of free radical pairs. Schulten’s team finally published the research results in 2000, pointing out that cryptochrome is probably the key molecule in the process of bird magnetic navigation, and boldly speculated on the magnetic induction process.
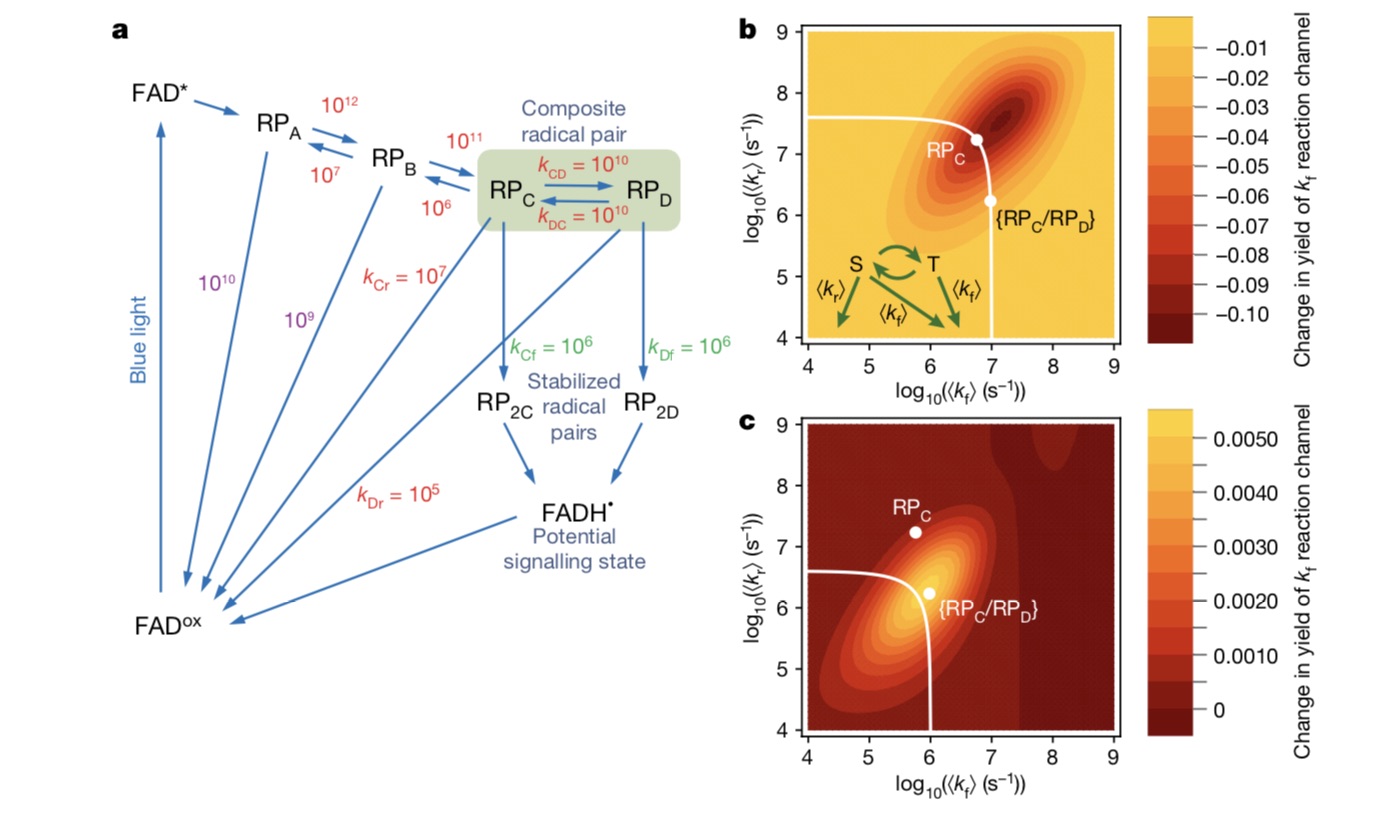
After nearly 20 years’ development, the outline of this hypothesis has become increasingly clear: firstly, the photosensitive pigment auxiliary group FAD in cryptochrome absorbs the energy of a blue photon, which causes the electronic transition of FAD group, leaving an empty orbit; Then the electrons are taken from the four adjacent tryptophan in turn, which is "electron transfer". At this time, the separated electrons have both spin singlet and triplet states due to quantum entanglement. Finally, the delicate balance between the two states can be determined by the direction of geomagnetic field, and this information can be transmitted to the brain in some way, so that organisms can respond to geomagnetic information.
The research team systematically and completely summarized the basic principles and supporting evidence of the free radical pair hypothesis from physics, chemistry and biology.
Compared with the previous theoretical research, this multi-party cooperative research takes the cryptochrome 4 protein of European robin as the main research object, carries out a series of high-precision spectroscopic tests, and carries out computational biology simulation, thus realizing the mutual verification of theory and experiment. This is the first comprehensive explanation of the photochemical magnetic sensitivity of free radicals in cryptochrome protein of nocturnal migratory birds, which confirms and perfects the magnetic induction mechanism of free radicals and provides an important basis for further explaining the geomagnetic orientation behavior of migratory birds.
Functional Cry4 protein from migratory birds
As early as 2000, scientists proposed that the photosensitive protein cryptochrome (Cry) was a possible magnetic induction protein through theoretical calculation. Theoretically, under the blue light condition, the information of external magnetic field can be transformed into the change of quantum yield caused by photoinduced free radicals in cryptochrome protein, which can be perceived by cells.
Why is cryptochrome protein considered by scientists as an animal magnetic induction molecule? The first author of this paper, Xu Jingjing from the University of Oldenburg, explained to the reporter of The Paper (www.thepaper.cn) that cryptochrome protein can bind to flavin adenine dinucleotide (FAD), also known as active vitamin B2. When FAD is excited by blue light, it will undergo a reduction reaction, which can seize the electrons of tryptophan (Trp) nearby in turn, thus forming a magnetically sensitive free radical pair [FAD-TrpH+].
"It can be said that FAD is the’ heart’ of this magnetic sensitive molecule, and only cryptochrome protein combined with the cofactor FAD has the prerequisite of magnetic sensitivity." Xu Jingjing is now a doctoral student in Henrik Mouritsen’s laboratory of the University of Oldenburg. Before studying in Germany, Xu Jingjing completed his master’s degree in the Institute of Electrician, Chinese Academy of Sciences, and received biochemical training in Professor Xie Can’s laboratory. .
It is worth mentioning that one of the reasons why the hypothesis of free radical magnetic induction has not been proved by experiments in birds since it was put forward in 1978 is that the recombinant expression of avian cryptochrome protein often loses its own function because of protein’s misfolding, which means the loss of the key auxiliary group FAD for cryptochrome.
"The biggest regret of free radicals to the hypothesis is that scientists have never been able to obtain experimental evidence of the magnetic sensitivity of cryptochrome protein in migratory birds. In the early attempts, some experiments were done in the laboratory with cryptochrome protein of plants or non-migratory animals, but as the most typical migratory animals, migratory birds have never been really studied because of the difficulty in purifying cryptochrome protein of birds. " Xie can added.
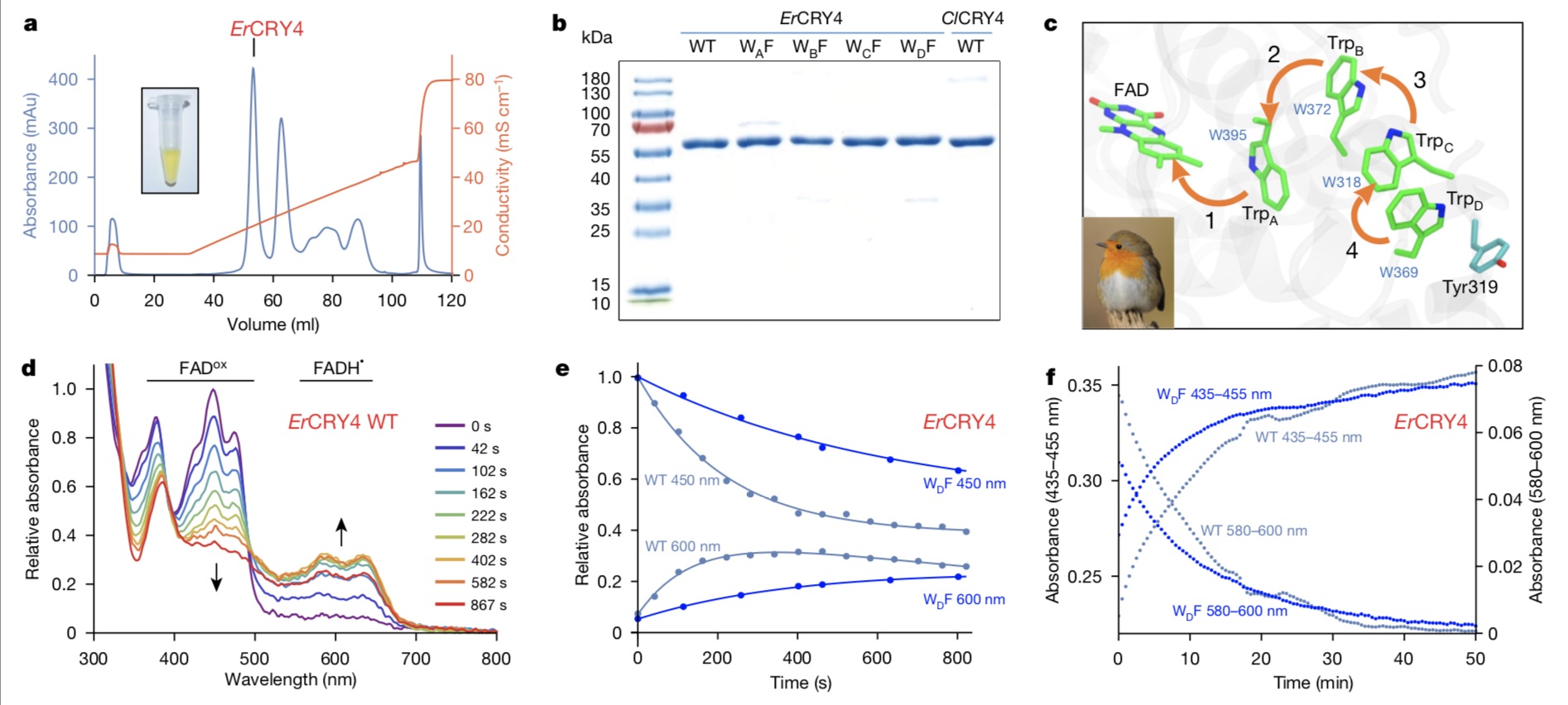
In this study, scientists finally obtained the functional cryptochrome protein of migratory birds that combined with FAD. Under the guidance of Xie Can and Mouritsen, Xu Jingjing expressed and purified Cry4 protein of nocturnal migratory birds with biological activity by using the Cold-Shock protein expression system customized and optimized in the laboratory. The protein sample showed a beautiful and transparent yellow-green color due to the presence of FAD chromophore, and mass spectrometry analysis showed that the FAD binding rate of the protein was as high as 97%. It laid the foundation for the success of the whole study.
The acquisition of Cry4 protein of European robin is actually quite tortuous. Xie Can told the The Paper reporter that as early as the beginning of the cooperation, that is, in April 2016, the original plan was that Xie Can’s laboratory would prepare the protein in China, and then send it to the foreign cooperation team by air transportation for the next test.
In the previous research on MagR, Xie Can has successfully expressed and purified the MagR and Cry proteins of homing pigeons (birds that can homing directionally but not migrate seasonally) and their complexes.
But the international transportation scheme is not feasible. Xie Can clearly remembers that on June 9, 2016, his laboratory sent samples to Oxford University for the first time, but it was difficult. "Long-distance international transportation of protein samples requires that the protein be frozen first, and then thawed after reaching the destination laboratory. At that time, we found a problem. Whether it is Cry protein, MagR protein discovered by ourselves, or a complex of the two, all these proteins related to magnetic induction are very unstable and extremely sensitive to temperature. In the process of freezing and thawing, the activity of protein is greatly reduced or even disappeared. " For Cry4 protein in this study, after long-distance transportation and freezing and thawing, the key FAD auxiliary group was dissociated from Cry4 protein, and Cry4 protein also lost its activity.
Under the circumstances at that time, how to "preserve" Cry protein was an urgent problem to be solved. Just then, Xu Jingjing was applying for a visiting scholar in Mouritsen Laboratory. In November 2016, Mouritsen put forward a proposal to let Xu Jingjing go directly to Xie Can’s laboratory to learn a whole set of protein expression and purification techniques. Under the framework of cooperation, Xie can is very open-minded. "This is also a good thing for us, because the articles we publish ourselves also hope that others can repeat them." After more than two months of intensive "crash course", Xu Jingjing entered the Mouritsen laboratory.
"It took about seven or eight months for Xu Jingjing to set up the whole system from scratch in Mouritsen laboratory, and the expression and purification of Cry4 protein were perfectly repeated, and it also had good activity." After the key cornerstone work was completed, Hore and Mouritsen could not hide their excitement. They expressed their excitement in the email sent to Xie Can, that is, at that moment, the three parties formally established a long-term cooperation. "The three of us are completely different backgrounds. Hore is a physicist, so he mainly does physical chemistry. Mouritsen does animal behavior and neurobiology. I do protein research, so the three of us have their own strengths, which can complement each other." Xie Can described this tripartite cooperation in this way.
In the following two years, Xu Jingjing usually finished protein preparation in the morning, then took a flight from Germany to Britain, arrived at Oxford University in the afternoon and went straight to the laboratory to test the properties of the protein, that is, the magnetic sensitivity of free radicals to photochemical reactions. Xie can once "experienced half a trip" when she visited Oxford University. "I can realize that she basically shuttled between laboratories in two countries for more than two years, and this is how the experiment was done." Xie can said.
For the first time, the hypothesis of free radical pair was verified by the experiment of cryptochrome protein of migratory birds
In the course of this research, in view of the complexity of the experimental content and the extremely complicated tests involved, more laboratories joined in. It is mentioned in the paper that in order to study the influence of magnetic field on the photochemistry of cryptochrome, the research team has developed and continuously improved a variety of spectral techniques.
The research team of Oxford University has developed a series of spectroscopic techniques specially used to study the photochemical magnetic field effect of cryptochrome, including transient absorption spectroscopy and Cavity ring-down spectroscopy. Broadband cavity-enhanced absorption spectroscopy and electron paramagnetic resonance. Hore described it this way, "These devices were developed by the joint efforts of generations of talented postdoctoral and graduate students in the laboratory for many years."
Xu Jingjing said, simply put, the researchers put the protein sample in a magnetic field and irradiated it with 450nm blue light to detect the light absorption changes of the protein transient free radical pair under different magnetic field conditions and different time resolutions.
The experimental results showed that the free radicals in cryptochrome protein changed the yield after the magnetic field was applied, that is, the magnetic field effect, and the magnetic field effect of cryptochrome protein of European robin was 10 to 20 times stronger than that of non-migratory birds (chickens and pigeons).
The research team also clarified the mechanism of this magnetic sensitivity, that is, the electron transfer reaction triggered by blue light absorption. Protein molecule is composed of a series of amino acids: Cry4 has 527 amino acids, of which 4 tryptophan is very important for magnetic sensitivity. Under the excitation of blue light, electrons jump between FAD and these four tryptophan, resulting in so-called magnetically induced free radical pairs. Peter Hore of Oxford University and Ilia Solov’yov, a physicist in oldenburg, have calculated quantum mechanics to support this view.
In order to verify this, researchers at the University of Oldenburg further prepared tryptophan mutant protein of cryptochrome, that is, four tryptophan (WA, WB, WC and WD) in the electron transfer chain were replaced by phenylalanine (F) in turn, which had no redox activity and could not provide electrons to FAD, thus cutting off the electron transfer chain.
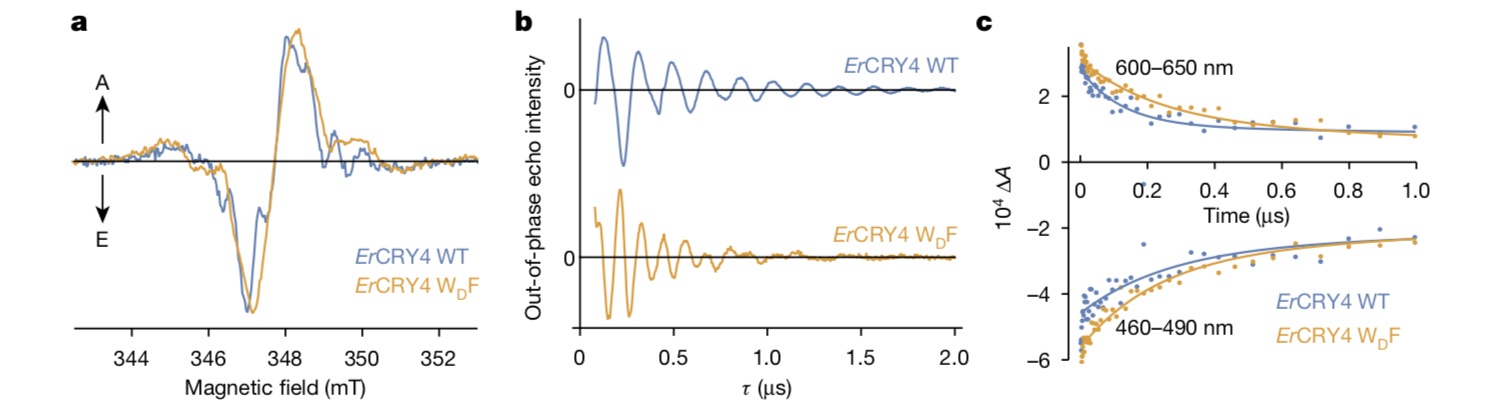
In each mutant protein, the electron transfer chain is 3, 2 and 1 tryptophan in turn. The transient absorption spectrum test results show that the free radical pair life of mutant protein is shortened from microsecond to nanosecond and picosecond. Electron paramagnetic resonance (EPR) test showed that the free radicals of wild-type protein [FAD+TRPDH+] were 2.2nm apart, and after the fourth tryptophan was mutated, the free radical pair [FAD+TRPCH+] was 1.8nm apart.
The paper points out that the experimental results are consistent with the theoretical simulation. In wild-type cryptochrome protein, electrons are transferred among four tryptophan in turn, and these four steps make the electrons that are not equipped at the end be the farthest apart, thus forming a long-lived free radical pair, and the ultra-fine effect of the latter’s spin coherence is the point of magnetic field action.
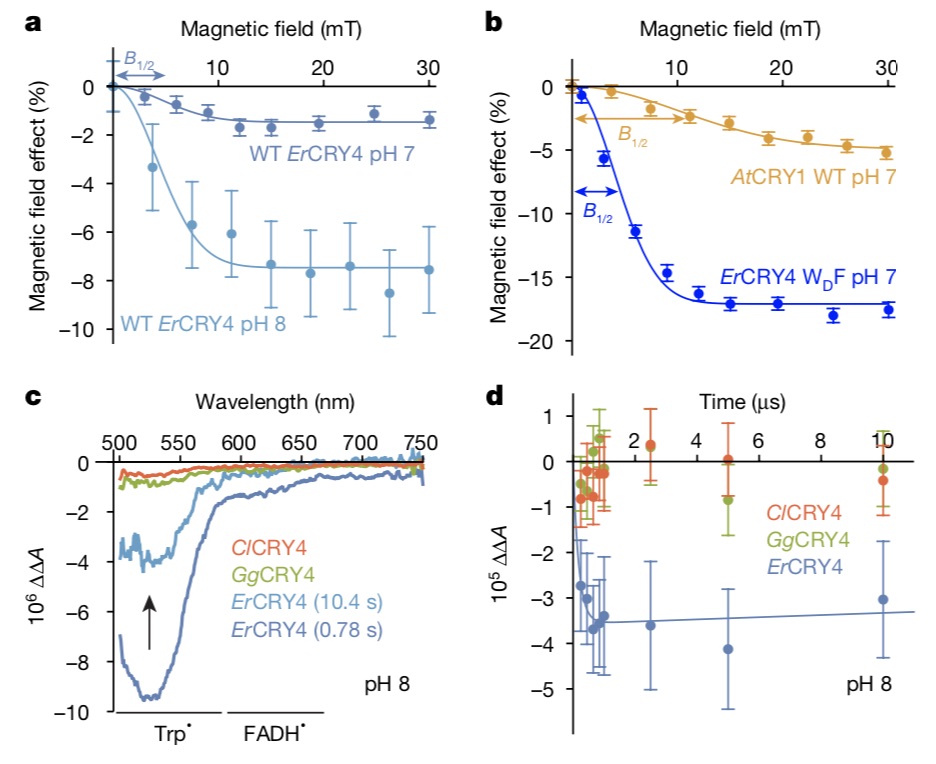
Xu Jingjing mentioned that, surprisingly, when the fourth tryptophan at the farthest end was mutated, the magnetic field effect of protein was stronger than that of wild-type protein, and it was stronger than that of cryptochrome in Arabidopsis thaliana, which is also an electron transfer chain of tryptophan.
The researchers asked, why did nature evolve a fourth tryptophan for avian cryptochrome protein, if its function was only to reduce the sensitivity of magnetic field? Further research shows that the decay kinetics of wild-type protein (ErCry4) and mutant (ErCry4 WDF) are similar in milliseconds, and the magnetic field dichotomy (B1/2) is similar. This means that the origin of their magnetic sensitivity is the same, both of them come from the third tryptophan, and the fourth tryptophan may not participate in the animal magnetic induction process.
So what role does this fourth tryptophan-mediated electron transfer play? Based on the above experimental results, Ilia Solov’yov and Hore put forward the mechanism of free pair dynamic equilibrium through spin dynamic simulation, that is, electrons may jump back and forth between the third and fourth tryptophan, and the resulting two free radical pairs [FAD-TRPCH+] and [FAD+TRPDH+] are in rapid dynamic equilibrium and play different roles respectively.
The two roles are: the free radical pair formed by flavin-third tryptophan is mainly responsible for magnetic field perception, and the free radical pair formed by flavin-fourth tryptophan is mainly responsible for biochemical signal transmission in vivo. "This mechanism has not been found in cryptochrome proteins of any species before." Xie can said.
The research team predicts that there may be other proteins interacting with cryptochrome proteins in the body, such as MagR or other unidentified signal molecules reported by Xie Can, to realize cascade amplification of signals or directional arrangement of cryptochrome proteins, and further enhance the magnetic sensitivity and direction recognition in organisms. "Of course, these speculations need more experiments in the future to confirm."
They speculated that, perhaps, it was through this ingenious mechanism that nature wonderfully optimized the free radical photochemical reaction of cryptochrome protein and realized the dual functions of magnetic field sensing and signal transmission, thus enabling migratory birds to find their way in their journey across the hemisphere.
Xie Can concluded to the The Paper reporter that the main breakthroughs of this study are as follows: first, the hypothesis of free radical pair is verified experimentally with cryptochrome protein of migratory birds; Secondly, it is found that cryptochrome protein 4 of migratory birds is more sensitive to magnetic field than that of non-migratory birds; Thirdly, it is found for the first time that the four conserved tryptophan in the hypothesis have two different functions: magnetic induction and signal transmission.
Moving forward in controversy, in vivo verification is not easy.
It is worth noting that there are still many controversies in the field of animal magnetic induction research.
Xie Can said that on the whole, the magnetic induction hypothesis that has attracted much attention so far mainly includes electromagnetic induction model, magnetite particle model, chemical free radical pair model dependent on cryptochrome and biological compass model. These hypotheses are supported by certain experimental evidence, but there are also some limitations or deficiencies.
Xie Can called the study of animal magnetic induction a "very non-biological" study. By analogy with the conventional research ideas of sensory biology, scientists have first known for sure the existence of a certain sensory system and what kind of sensory organ it is, and on this basis, they have identified the receptor cells, neural pathways and brain regions related to the sensory system.
But the study of magnetic induction is quite different. In this field, biologists often find and ask questions, but physicists put forward imaginative hypotheses, and then chemists will also participate in a large number of verification experiments. Previously, the discovery of MagR and the hypothesis of biological compass in Xie Can’s laboratory generally followed this thinking paradigm.
Although this latest research has made many important breakthroughs, Hore stressed that this is not conclusive evidence that cryptochrome is a magnetic sensor. Because "this study is an in vitro experiment with purified protein, and the magnetic field used in the experiment is much higher than the geomagnetic field." Henrik Mouritsen said, "We need to prove the sensitivity of this protein to geomagnetic field in birds’ eyes, but the current technical bottleneck does not allow us to do this."
The research team speculated that cryptochrome protein may show higher sensitivity to magnetic field in vivo environment. For example, in retinal cells, protein may be arranged in a fixed orientation, thus enhancing their sensitivity to the direction of magnetic field. In addition, they may interact with other proteins, such as the previously reported magnetic receptor MagR, and even other unidentified protein, so as to achieve the effect of signal cascade amplification. Scientists are also looking for these unknown "mutual partners".
Regarding the future research plan of this project, Xu Jingjing pointed out that finding the interaction protein of cryptochrome and conducting in vivo experiments are two major challenges.
In fact, up to now, even though the evidence of free radicals is the most comprehensive, many hypotheses of animal magnetic induction mechanism need to be continuously developed, improved and screened. "There are many schools in the field of magnetic induction, and the publication of every research result needs to be widely questioned and challenged by reviewers in the review process, and these reviewers often come from different academic schools with different academic viewpoints, and they are often your competitors. Only by persuading your competitors will it be possible to finally publish it through review. " The review of this paper for nearly two years also highlights the twists and turns of the process.
Xie can prefers to call this kind of controversy in the scientific community academic debate. "The extensive academic controversy and debate itself shows that the problem you are studying is very important, which is obvious." Secondly, Xie Can believes that with the academic controversy and debate, it often brings about the rapid development of the whole field, "because more and more rigorous experiments will be designed to verify their respective views. Whether it is proof or falsification, we are getting closer and closer to the truth of science. "
Xie Can believes that the field of animal magnetic induction is full of controversy, "because no hypothesis or model has been recognized by everyone so far, and each school has its own supporters."
Strong cooperation like this one may bring about accelerated progress. "This is a highly interdisciplinary field. If you want to do some interesting topics in the field of animal magnetic induction and biological navigation, it is often impossible to rely on a laboratory to complete it." Xie can said.
Xu Jingjing also mentioned that "solving a scientific problem requires the joint efforts of many disciplines. In our team, experts in various disciplines have world-class technology, which is very important for our research." She said that in the process of project promotion, researchers kept communicating and understanding the knowledge of different disciplines to achieve common scientific goals.
关于作者